Introduction
A Sirona sensor-based fluorescence lifetime imaging (FLIM) line-scanning microscope is described, developed by Dr. Simon Poland and colleagues at King’s College London and the University of Edinburgh (Mai et al., 2023). The microscope utilises a laser-line focus optically conjugated to a 1024 channel Sirona single-photon avalanche diode (SPAD) sensor. High-speed FLIM performance in biological applications is presented.
Materials and Methods
Sirona supports in-pixel CMOS construction of time-resolved histograms in each of up to 1024 channels, with up to 50ps time resolution. The FLIM microscope utilizes a laser-line focus optically conjugated to the Sirona line-sensor, and incorporates a cylindrical lens to generate a laterally stretched Gaussian intensity distribution beam. A single galvanometer scanner with relay optics is used to build up successive line images on the sensor to form a composite 2D image.
Optical System
The time-resolved optical microscope built around a Nikon Eclipse Ti inverted microscope incorporating the Sirona SPAD sensor is shown in Fig. 1. Frequency-doubled laser light from a Ti:sapphire laser system yields a 490nm wavelength output which is expanded and relayed onto a cylindrical lens to generate the line beam. The beam passes through a dichroic filter onto a scanning mirror and scan and tube lenses to the back pupil of an objective lens. A sweeping line scan is thereby generated directly on the sample plane. Fluorescence is collected, de-scanned, and then reprojected onto the Sirona linear array sensor. A high-speed data acquisition device synchronizes the scanning galvanometer position with the data acquisition from the Sirona linear sensor. For each scanning step the Sirona acquires photon arrival times to generate a 32-bin histogram in each pixel. At the end of the exposure cycle, histograms are sent to the computer. Following line sweeps across the sample, the fluorescence is collected and reconstructed into a 512×512×32 dataset. The system can capture image data at a single plane or at multiple depths. A motorized stage on the Nikon Ti microscope facilitates high-speed acquisition of tiled mosaic FLIM images at macroscale.
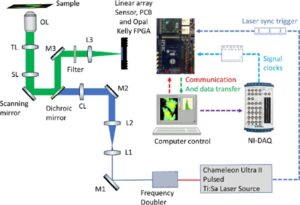
Fig. 1 FLIM Microscope (Mai et al 2023)
Fluorescence Lifetime Imaging
Full details of FLIM experiments carried out with the system can be found elsewhere (Mai et al., 2023). Sirona’s in-pixel time-correlated single-photon counting (TCSPC) histogramming enabled acquisition rates 33 times faster than the group’s previously reported high-speed FLIM systems. In Fig. 2 we present high-speed macroscale images acquired on the order of seconds. To generate a 2 mm×2 mm mosaic in 49 seconds, 14×14 (196) images were acquired at acquisition times of 250 ms. The mouse lung H&E macro image was acquired in 12.5 s and is composed of 7×7 individual images.
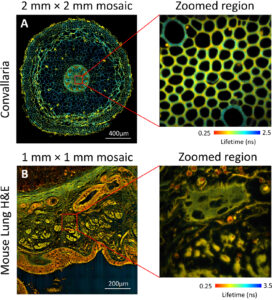
Fig. 2 Macroscale FLIM composite images of (a) Convallaria majalis (7168 ×7168 pixels) acquired in 49 s and (b) a mouse lung H&E sample (3584 ×3584 pixels) acquired in 12.5 s. Zoomed regions of single image tiles (512 × 512) are also included. (Mai et al., 2023)
Conclusion
These FLIM experiments demonstrated that line generation and laser-line scanning in conjunction with on-chip histogramming on a Sirona linear SPAD array sensor is practical and fast and offers novel advantages for fluorescence lifetime imaging.
Acknowledgements
Funding for this work was supported by UK Research and Innovation (MR/T04067X/1); Engineering and Physical Sciences Research Council (EP/K03197X/1); and the Royal Society (IES\R3\183065).
References
Copyright
This document ” Sirona Sensor-based Line-scanning Fluorescence Lifetime Imaging Microscope ” is adapted from (Mai et al, 2023) and licensed under CC BY 4.0
Mai, H., Jarman, A., Erdogan, A. T., Treacy, C., Finlayson, N., Henderson, R. K., & Poland, S. P. (2023). Development of a high-speed line-scanning fluorescence lifetime imaging microscope for biological imaging. Optics Letters, 48(8), 2042. https://doi.org/10.1364/OL.482403