Introduction
The Andarta Sensor from Singular Photonics is a 512×512 single-photon avalanche diode (SPAD) array (Gorman, 2024). Incorporating several photon acquisition modes implemented in the latest 3D-stacked CMOS technology, a stand-out feature of Andarta is support for diffuse correlation spectroscopy (DCS) in ensemble and imaging configurations. Andarta DCS characterises motion of scattering particles such as red blood cells in tissue, e.g. for cerebral blood flow monitoring applications, providing important indicators of brain health. Configured in a 128×128 macropixel format, highly parallel in-pixel autocorrelation computation is supported, with a minimum autocorrelation lag-time less than 1 μs. As a result, Andarta can resolve changes in body tissue decorrelation in real time, at the longest source-detector separations achieved by the current leading optical modalities for cerebral blood flow monitoring.
In this Research Highlight we report the work of Prof. Robert Henderson (Chief Scientist at Singular Photonics), Dr. Alistair Gorman and colleagues at the Institute for Integrated Micro and Nano Systems, University of Edinburgh to characterise direct, on-chip computation of the autocorrelation function of the Andarta sensor. Suitability for in-vivo data acquisition is illustrated with cuff-occlusion measurements.
Materials and Methods
The Andarta sensor uses advanced 3D-stacked backside illuminated (BSI) SPAD technology to provide enhancements in detection efficiency and to enable densely populated logic layers to deliver embedded pixel-parallel computation of autocorrelation. The entire macropixel array employs 120 M transistors, supports 0.5 T MAC operations per second (Op/s) and consumes around 0.3 W power.
Andarta macropixels calculate autocorrelation components directly during each integration cycle, rather than streaming photon-counting frames for off-pixel autocorrelation computations. Averaging of individual macropixel autocorrelations is supported in an on-chip, high-speed “ensemble” mode yielding strong signal-to-noise-enhancement for multispeckle DCS applications.
Optical System
The optical setup used for diffuse correlation spectroscopy with the Andarta SPAD sensor is shown in Fig. 1. A stabilized laser diode (Thorlabs LP785-SAV50) is driven in continuous wave mode. The laser is collimated and attenuated as necessary. Light transmitted through the sample is collected with a fiber bundle, consisting of seven 550-μm core multimode fibers, and relayed to the Andarta detector array.

Fig. 1 Optical Setup for Diffuse Correlation Spectroscopy (Gorman et al 2024)
Diffuse Correlation Spectroscopy
Ensemble mode DCS measurements demonstrate the ability of the sensor to resolve changes in blood flow in cuff occlusion measurements. Cuff occlusion measurements are taken at the ulnar side of the left palm (Fig. 2(a)), with a laser to detection fiber separation of 40 mm. Measurements are taken during and after a gradual, approximately linear increase of occlusion pressure of the left wrist, starting at 0 mm Hg and reaching a maximum of pressure of 165 mm Hg at 40 s, at which point there is an abrupt decrease to 0 mm Hg.
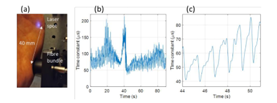
Fig. 2 (a) Source and detection fiber at palm. (b) Time constant during and after a linear increase of wrist occlusion pressure from 0 mm Hg to a peak of 165 mm Hg at 40 s. (c) Six pulse periods of the time constant post occlusion. (Gorman et al 2024)
Figure 2(b) shows a plot of the time constant from exponential fitting of the autocorrelation values. The time series shows the characteristic post-occlusion reactive hyperemia (higher blood flow). Also shown in Fig. 2(c)) is an example of six periods of the pulse waveform post occlusion.
Measurements of cardiac signals from the forehead of an adult subject, diffuse correlation imagery and full details on the Andarta DCS performance can be found in the journal article (Gorman et al. 2024).
Conclusion
In-vivo DCS measurements demonstrates use of the Andarta sensor to show sensitivity to changes in blood flow. The ability to measure signals in tissue with large source-detector separations highlights the potential of this sensor for non-invasive monitoring of cerebral blood flow. In-pixel and on-chip real-time calculation of the autocorrelation function eliminates the need for post-processing, reduces computational burden and enables faster data acquisition rates. Tiling of Andarta arrays to further SNR improvements, and provision for simultaneous measurements at multiple locations is readily supported with our architecture. Compact size and low power consumption of Andarta make it an attractive option for wearable or portable devices.
Acknowledgements
The Engineering and Physical Sciences Research Council (EP/T020997/1) and Meta Platforms Inc. are thanked for funding this work.
References
Copyright
This document “Diffuse Correlation Spectroscopy with the Andarta Sensor ” is adapted from (Gorman et al, 2024) and licensed under CC BY 4.0
Gorman, A., Finlayson, N., Erdogan, A. T., Fisher, L., Wang, Y., Mattioli Della Rocca, F., Mai, H., Sie, E. J., Marsili, F., & Henderson, R. K. (2024). ATLAS: a large array, on-chip compute SPAD camera for multispeckle diffuse correlation spectroscopy. Biomedical Optics Express, 15(11), 6499.